Understanding ‘Wild Horse Fire Brigade’

As discussed in the compelling article published below, “Wild horses that are restored back into their evolutionary roles as keystone herbivores naturally protect forests, wildlife, watersheds and wilderness ecosystems, which benefit through symbiotic grazing by wild horses that naturally maintain wildfire fuels (grass and brush) to nominal levels, thereby reducing the both the frequency and intensity of wildfire.”
<!–more—>
Note: this post appears with permission of the author – William E. Simpson II. More content can be found on his site at: https://www.wildhorsefirebrigade.org
Understanding ‘Wild Horse Fire Brigade’
The Supporting Science of Wildfire Grazing
Catastrophic wildfires are incinerating our forests. Wildlife caught in these infernos are being killed by millions.
Post-wildfire erosion is responsible for disastrous debris-flows and the silting-in of critical spawning grounds for migratory fish and trout, devastating fisheries.
And even though massive budgets for wildfire suppression and other heavy fuels management methods have doubled and tripled over the past decade, the number of annual wildfires is virtually doubling year over year, as well as their size and intensity.
As a result, the empirical results and logic dictates that existing methods for preventing and reducing the adverse impacts of wildfire are failing.
To understand why, and how this devastating result can be changed, we must first examine some key factors for this ongoing management failure.
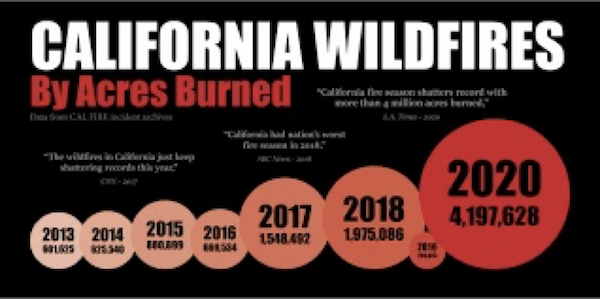
Image courtesy CAL-FIRE.
Why do wildfires burn?

Three elements are needed for a fire to start and continue to burn:
- fuel (dry grass, wood, brush)
- oxygen (from the air)
- ignition source (heat from lightning or man)
If any one of the three foregoing elements is missing, there can be no fire.
The basic principle of fire-prevention and/or wildland firefighting is to remove one or more of these elements in the quickest and most cost-effective way.
Forensic data reported at InciWeb- Incident Information System (https://inciweb.nwcg.gov) for hundreds of recent wildfires clearly shows that flashy wildfire fuels (grass and brush) are key fuels in a majority of wildfires.
Grass and brush fueled wildfires are fully capable of incinerating homes surrounded by concrete sidewalks and streets (Tubbs Fire, Sonoma Fire). Grass and brush fuels offer prime fuel for ignition and are super-hot-burning. Additionally, where prodigious grass and brush fuels are driven by wind, they quickly carry fire to heavier fuels, such as forests and structures, as recently seen in Boulder Colorado.
In Big Sur, winter rains produced a bumper-crop of un-grazed grass and brush, which fuels a devastating wildfire:
“Although El Niño rains last winter gave Northern California a reprieve from the drought, they didn’t completely offset the dryness deep in the soil and spawned more grass and brush growth, increasing wildfire risk.”
Empirical evidence suggests that many of these kinds of grass and brush fueled wildfires could have been prevented, or minimized via the implementation of an herbivory (using grass and brush eating mammals), which reduce these fuels and maintain them year-round.
A significant body of published peer-reviewed science suggests that; when populations of large-bodied herbivores become depleted on any landscape, throughout history, catastrophic wildfire evolves. [1, 2, 3, 4]
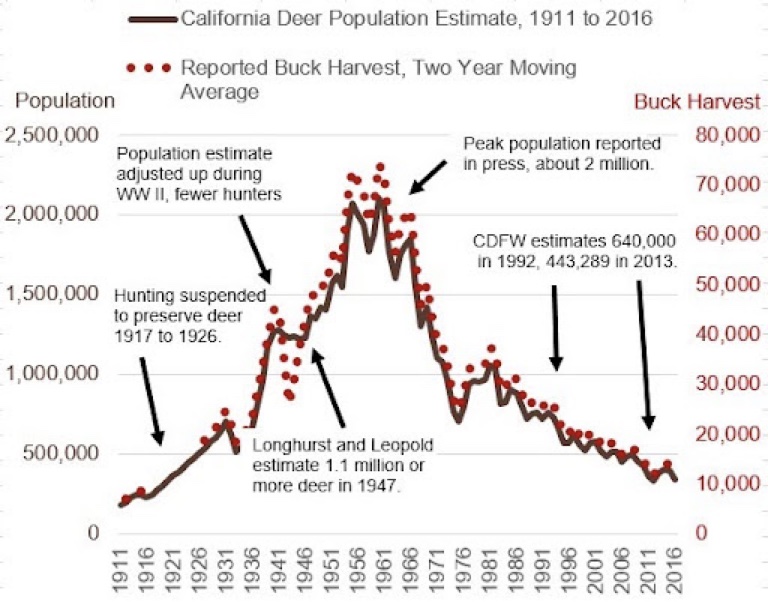
Wildlife data from 2016 shows California’s deer population has dropped about 1.5 million animals over the preceding 5-decades. And between 2016 and 2022, we estimate another million deer have been killed due to wildfires, incidents with vehicles, hunting and disease. The result suggests California is now down about 2.5million deer.
This is significant in that each missing deer was consuming about 7-pounds of grass and brush per day, or about 2,500-pounds of grass and brush annually per deer.
Using the foregoing math, the now missing 2.5-million deer are no longer reducing wildfire fuels at the rate of 3.2-million tons of grass and brush fuels annually in California.
Oregon is also suffering from a seriously depleted native species herbivory.
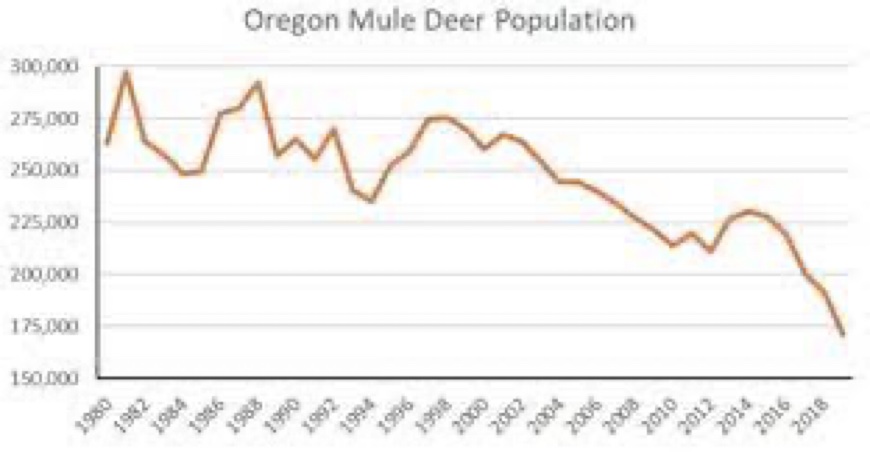
The graph above shows that Oregon’s mule deer population is down about 125,000 animals over the past 4-decades, and this graph doesn’t account for the added drop in black-tail deer populations of about 150,000 black-tail deer. The larger mule deer consume about 10-pounds of grass and brush daily, compared to 7-lbs./day by black-tail deer. These now missing mule deer had been consuming about 228,000-tons of grass and brush annually in Oregon. The depleted black-tail deer in Oregon estimated to be down by 150,000 animals were consuming about 192,000-tons of grass and brush. As a result, it is arguable that approximately 420,000-tons of annual grass and brush wildfire fuels are no longer being mitigated in Oregon.
Western forests are currently being wiped-out by catastrophic wildfire, which involves the impacts of grass and brush fuels. Currently, forests in the western United States are being destroyed by wildfire at a rate that far exceeds their recovery. The loss of these forests by catastrophic wildfire is accompanied by the death wildlife by the millions.
Anywhere from 20-80 animals are killed for every acre of forest burned. And the post-wildfire erosion presents its own disastrous impacts.
The secondary effects of catastrophic wildfire include erosion, landslides and debris-flows and changes in water quality that are often more disastrous than the wildfire itself.
Many wildfires are preventable and there is a tool that is not being used
There is an important hypothesis that offers an ecologically beneficial and cost-effective solution in the battle to mitigate the frequency and intensity of wildfire.

A family band of wild horses has symbiotically grazed-in a natural fire-break that protects an old-growth forest and the wildlife therein.
The Natural Wildfire Abatement and Forest Protection Plan (the ‘Plan’) is premised upon a hypothesis that is supported by a case study conducted by William E. Simpson II in the area of the Soda Mountain Wilderness Area of the mountains on the Oregon California border, commonly called ‘Wild Horse Fire Brigade’.
Wild Horse Fire Brigade helps mitigate wildfire by restoring native wild horses as keystone herbivores into designated wilderness areas rich with forage and water where they benefit flora and fauna as they reduce and maintain grass and brush wildfire fuels, beyond conflicts with livestock and other public land uses.
More here (video): ‘Fuel, Fire and Wild Horses’ https://vimeo.com/327282987
Wild Horse Fire Brigade is about helping to save forests and wildlife, as well as saving native species American wild horses by rewilding them from government holding facilities, and/or relocating them away from areas of contention with livestock production. This new Plan, seeks to humanely place wild horses as family units into carefully selected designated wilderness areas that are economically and ecologically appropriate, where they will reduce and maintain grass and brush fuels to more natural levels.

This photo shows an area in the BLM Herd Management Area (Pokegama) which burned during the Oregon Gulch Fire in 2014. The remaining burned trees (dry, pitch-filled wood) are now ready to re-burn even hotter than before. The grass and brush have once-again filled-in and around the dead trees and will assure a future catastrophically hot wildfire. The BLM’s so-called ‘appropriate management level (AML) of 30-50 wild horses in the area (80,885 acres) assigns each horse to approx. 2,000 acres, in an area with a depleted cervid population.
There is approximately 110-million acres of designated wilderness area in America, primarily in the western United States, and in such areas, motorized vehicles and equipment are prohibited by law. These designated wilderness areas have abundant forage and water resources, but are manifestly unsuited for livestock grazing due to existing law, existing populations of apex predators and excessive logistics and transport costs due to the difficult terrain and remoteness of such locations.
In such wilderness areas, wild horses that are restored back into their evolutionary roles as keystone herbivores naturally protect forests, wildlife, watersheds and wilderness ecosystems, which benefit through symbiotic grazing by wild horses that naturally maintain wildfire fuels (grass and brush) to nominal levels, thereby reducing the both the frequency and intensity of wildfire.
The goal of Wild Horse Fire Brigade is to naturally and sustainably save America’s remaining native species wild horses by establishing several experimental wilderness wildfire-grazing pilot programs using rewilded/relocated American wild horses that are sourced from government agencies using existing law (Humane Transfer of Excess Animals – Wild Horses).
https://www.blm.gov/policy/im-2018-052
Another goal is to also support the amendment of Section 1339 of the 1971 Free-Roaming Wild Burro and Horse Protection Act, to allow federal managers to also directly rewild and relocate wild horses into ecologically and economically appropriate designated wilderness areas outside existing herd management areas (HMAs).
Top-12 Benefits of ‘Wild Horse Fire Brigade’

A family band of native species American wild horses – Photo: William E. Simpson II
1. Saving Wild Horses:
Wild Horse Fire Brigade provides sustainable cost-effective natural conservation of native species American wild horses by rewilding-relocating them into designated wilderness areas that are economically and ecologically appropriate, which have abundant forage and water, where wild horses are restored to their evolutionary roles as north American keystone herbivores. In such areas, wild horses are no longer commingled with livestock, which eliminates the political and economic pressures that are currently being applied to native species American wild horses.
More here: Economics vs. Wild Horses https://www.sierranevadaally.org/2021/12/23/comsumerism-vs-wild-horses/
2. Rewilding Benefits:
Rewilding American wild horses from ‘Herd Areas’ and ‘Herd Management Areas’ where they are currently mismanaged via being commingled with livestock, and relocating wild horses into designated wilderness areas that are both ecologically and economically appropriate, is a genetic benefit for wild horses.
Over the past centuries, many herd areas have been managed specifically for livestock production and have by design been made virtually devoid of apex predators, making such areas ecologically unsuited for wild horses.

This young colt (~18-mos. old) was taken at neck by a mountain lion. Over a period of a few days, other predators (bears, coyotes, golden eagles) scavenged the carcass. Photo: William E. Simpson II
Wild horses (E. Caballus) evolved in North America 1.7-million years ago, and are a prey species that require co-habitation with their co-evolved predators. Apex predators engage in the process of Natural Selection that preserves the genetic vigor of wild horses. The predators of wild horses (bears, mountain lions, wolves and coyotes) take the weak, sick and elderly animals, which preserves the overall genetic health and vigor of wild horse herds, while also managing populations to nominal levels, thereby negating the great expense of the ill-conceived notion that contraceptives should be used on American wild horses.
The use of any form of contraception (chemicals to sterilize mares or castration of stallions) is by definition ‘selective breeding’ and leads to genetic erosion. Such actions are currently being used as a misguided work-around due to the lack of apex predators, and interferes with the critically important processes related to the behavioral ecology of wild horses. Stallions must be allowed to compete for breeding rights to mares, and a mare’s hierarchy in a family band is partly determined by her sexual status and ability to procreate, which stallions and other mares can sense.
More here: Selective Breeding of Wild Horses Accelerating Genetic Erosion https://rtfitchauthor.com/2021/12/17/selective-breeding-of-wild-horses-accelerating-genetic-erosion/
Finally, the relocation (rewilding) of wild horses from herd areas frees-up more grazing for livestock in areas that are virtually devoid of apex predators. The combination of the foregoing is a win-win for the wild horses and the livestock interests, and ends the longstanding and very costly range war.
More about the range war: ‘Wild Horse Wars’ https://www.sierranevadaally.org/2021/04/05/wild-horse-wars/
3. Massive Savings for Taxpayers:
Putting wild horses back into the wilderness where they belong immediately saves American taxpayers over $150-million in annual costs related to the Bureau of Land Management’s and the United States Forest Service’s inhumane, wasteful and unreasonable management, helicopter roundups, and off-range holding and feeding (warehousing) of American wild horses. An added benefit is the reduction of wildfire fuels via wild horses ecologically appropriate wildfire grazing, which published science and empirical data prove, will reduce the frequency and intensity of catastrophic wildfire.
If such reductions are even in the tiny realm of 2-3% across the western landscape annually, when calculated against wildfire losses and costs that are nearly $100-billion/yr., the added saving to taxpayers amounts to about $30-million dollars. As a result, the combined saving to taxpayers is about $180-million annually.
4. Wildfire Fuels Reduction and Maintenance:
Wild horses deployed into designated wilderness areas, where motorized vehicles and equipment and prescribed burns are generally prohibited by law, naturally reduce and maintain hot-burning grass and brush fuels to nominal levels. Thus, according to the leading science today, this action reduces both the frequency and intensity of wildfire.
More here: ‘Wildfires and Wild Horses’ https://www.linkedin.com/pulse/wildfires-wild-horses-whats-connection-william-e-simpson-ii
Grass and brush fuels reduced and maintained by wild horses also reduces the potential adverse effects of overheating soils and thereby, destroying the microbiome, to name just one adverse effect.
More here: Low-severity wildfires impact soils more than previously believed – Negative effects of low-severity fire on soil structure and organic matter
https://www.sciencedaily.com/releases/2018/09/180910160632.htm
Given the horrific costs in human life, adverse health impacts and climate impact from billions of tons of toxic smoke, loss of homes in the thousands annually, and the insured and uninsured losses, which are in the $-billions annually, even a small reduction in wildfire results in additional savings in the realm of hundreds of $-millions annually.
5. Natural Reseeding of Native Plants:

Horse dung (photo above) contains undigested seeds of native plants together with mico-biome that provides beneficial reseeding of native plants and restores fire-damaged soils. Photo: William E. Simpson
Unlike invasive species ungulates (cattle & sheep), wild horses have a simple digestive system that scientific studies show, do not digest the majority of the native plant and grass seeds that wild horses consume. Therefore, even as wild horses are reducing wildfire fuels via grazing, wild horses concurrently reseed the landscape via the intact seeds that are deposited back onto the landscape and able to germinate in their droppings.
6. Forests Benefited:

The juniper tree in the photo above was used for shelter by wild horses (note horse droppings). Flames from the Klamathon wildfire burned right under this tree, yet it survived having been made fire-resilient by the combined symbiotic actions of wild horses, allowing this tree to survive where other nearby trees were not used by wild horses became matchsticks! Trees that were being used by wild horses as shelters had the fuels (grass and brush) under their canopies grazed down so flame heights under trees were greatly reduced. And these large-bodied herbivores (wild horses) scratch themselves using the lower dead branches on these trees (aka fire-ladders), breaking them off. The aggregate result of this symbiotic behavior (tree provides shelter, horses provide fertilizer, and fuels reduction making trees fire resilient) saves trees and shows how wild horses are the protectors of forests and wildlife. There is no doubt that the local wild horses helped to save the precious Soda Mountain Wilderness Area from the deadly Klamathon Fire of 2018. – image: William E Simpson II
Wild horses have co-evolved using trees as shelters during all seasons. This symbiotic relationship benefits the horse with shelter and benefits trees because horses graze the grass and brush fuels under the trees they use as shelters, break-off low limbs (aka: fire ladders) and fertilize trees with their droppings, all of which make trees more fire resilient.
More at GrazeLIFE: https://grazelife.com/blog/wild-horse-fire-brigade-lessons-in-rebalancing-north-american-ecosystems-by-rewilding-equids/
7. Eco-Tourism:
Wild horses are American icons and treasured by over 100-million Americans. This love of American wild horses drives ecotourism in areas that have free-roaming wild horse herds, which helps bring revenues and jobs into such areas.
More here: https://ecotourism.org/news/ecotourism-a-path-towards-better-conservation/
8. Sequestering Carbon:
Wild horses that graze grass and brush fuels are sequestering carbon compounds back into soils in their dung, which also incorporates the seeds of native plants, humus and microbiome, all of which restore and enrich soils, including areas with wildfire-damaged soils.
9. Reduces Need for Prescribed Burning:
Wild horses naturally grazing reduces the need for excessive prescribed burning of grass and brush, which sends millions of tons of carbon compounds into the atmosphere, accelerating climate change and ocean acidification. This also reduces the amount of toxins that impact air quality creating serious health issues.
More here: Intelligent Forest And Wildland Management Reduces Catastrophic Wildfire
And here: https://pagosadailypost.com/2021/05/07/opinion-species-collapse-wildfires-ocean-acidification-is-there-a-link/
10. Soil Disturbance:

A small herd of native wild horses made-up of several families. This alpine riparian area has been documented as being used by wild horses for centuries, yet remains pristine. Photo: William E. Simpson II
As discussed in Section-5, the biology of a candidate herbivore being considered for any herbivory project is critically important.

The herd of wild horses in the prior photo are using this stream from water, which is centered in the alpine riparian meadow. Even with over 50-wild horse using this water resource, the turbidity of this stream is low. Photo: William E. Simpson II
So too, the morphology of any candidate herbivore is an important factor in selecting the proper herbivore for a particular area targeted for wildfire grazing.
Teeth And hooves matter when choosing an herbivore for ecologically sensitive areas.
Often overlooked is the biology and morphology of the large-bodied herbivores that are grazing public lands and wilderness areas. These factors are critically important in regard to intelligent land management, especially in ecologically sensitive areas, and for the restoration of wildfire damaged wilderness lands.
Unlike cattle, sheep and goat, which only have bottom front teeth, wild horses have top and bottom teeth (incisors), which cleanly cuts through the vegetative materials they graze.
Because cattle have no top front incisors, they have a long prehensile tongue that is used to wrap-around and help pull grasses over their bottom front teeth, cutting like a scythe. The result is that cattle tend to uproot some plants and grasses as they graze.

All horses have upper and lower front incisor teeth.
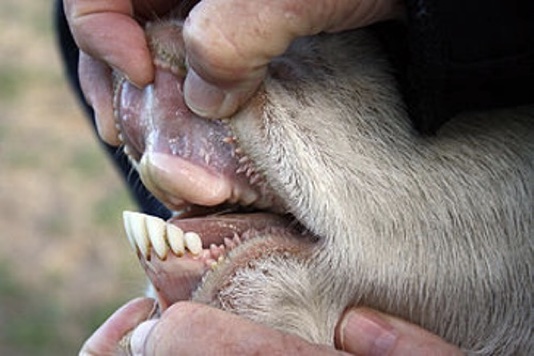
Cattle, sheep and goats have no upper front incisor teeth.
Hoof Anatomy: The impact on post-wildfire soils
The evolved hoof of a native species American wild horse has a much greater surface area in proportion to body weight than do invasive species cattle.
After 55-million years of evolution on the North American continent, the horse’s hoof is evolved to be in perfect ecological harmony with the North American landscape.
NOTE: The photos below show cow claw and horse hoof penetration depths in a native pasture. They were all taken in the same immediate area (by the author) where cattle and horses had walked in a native pasture on the same morning.
Here are the simple mechanics of the evolutionary hoof design (difference) between of horses of North America, and cattle (evolved in Africa), and how each design uniquely impacts the soils in our North American forests, range-lands and riparian areas.

This is a typical cow hoof (actually, it’s a bifurcated claw).
The photo above shows the underside of a cow’s hoof, which as we see, has what are termed as pointed claws. Like the point of a pick-axe tool, the points on these cow claws are very effective at penetrating deeply into soils, especially soft or wet soils. We also note that these claws present a relatively small surface area (pounds/square inch loading) upon which the entire weight of the cow is supported. And this point is obvious on its face.

Cow’s hoof penetration into soil of a native pasture is about 5 inches. Photo: William E. Simpson II
The photo above is an example of what a cow’s claw does in soft pasture as a result of the shape and high ground loading (weight/surface area of claws). As we see, the cow that made this imprint (weight est. 800-900 pounds) penetrated the soil at least five inches deep.
Now we turn to the design and effect of the horse’s hoof on soils.

The underside of a horse’s hoof.
In the photo above we see the underside of a horse’s hoof. It is distinctly different from the cow’s claw in many ways that protect the soils where they tread. First off, the shape is as we see rounded and the surface area is relatively large (lower ground loading in pounds/square inch). The hoof is dished-in on the underside (concave) and that shape tends to trap water and air under the hoof and allows it to ‘float’ hydraulically on the soil instead of piercing the soil like the pick-shaped cow claw.
As we see, the hoof has a lot of surface area compared to the cow claw, and that greater surface area distributes the weight of the horse over more area on the soil, which limits the penetration of a horse’s hoof into the soil. Thus, we have less soils disturbance via the significantly lower ground loading as measured in pounds per square inch (PSI).

The horse hoof makes an impression in the soil of less than an inch deep. Photo: Simpson
In the photo above we clearly see the horse hoof makes an impression in the soil of less than one-inch deep (same area/soil which contained cow imprints). The horse that made this imprint in the photo above weighed about 800-900 pounds (similar to the weight of a cow), which is a typical weight for an adult wild horse.
Wild horses evolved in North America 1.7-million years ago and are a co-evolved species to other North American flora and fauna. Even their hoof design is unique, which results in low-impact on soils. The body-weight of a horse, when calculated over the surface area of their hooves, yields a significantly lower ground-loading and compaction (in pounds per square inch; PSI) as opposed to cattle. Cattle have less hoof surface area in proportion to their body weight, which causes significant disruption of soils, which is bad for wilderness ecosystems. Therefore, the best species of herbivore for wildfire-grazing in ecologically sensitive designated wilderness areas is without doubt the wild horse.
More here: Evolution of wild horses and cattle and the effect on range damage https://www.horsetalk.co.nz/2017/09/25/evolution-wild-horses-cattle-effect-range-damage/
And here: https://pagosadailypost.com/2021/01/18/opinion-wild-horses-chronic-wasting-disease-in-deer/
11. Erosion, Flora, Fauna and Fisheries:
Wild horses naturally maintain native species cover crops, which are important to the survival of co-evolved dependent fauna. Science shows that maintaining cover crops also helps to prevent soil erosion. The opposite is true when fire (prescribed or natural) strips-off a cover crop resulting in high levels of erosion and loss of water infiltration into aquifers. Post-wildfire erosion in late fall and early winter causes abnormal erosion of clay and silt sediments that damage the spawning grounds and cover fish eggs, which adversely impacts native species fisheries. Excess sediment can profoundly impact the productivity of a salmon or trout stream (Cordone and Kelly, 1961)
More here: Excess Sediment https://www.krisweb.com/stream/sediment.htm
12. Reestablishing Deer and Elk Populations:
Published peer-reviewed science shows that wild horses and deer are ‘commensal’, which means they don’t take forage from each-other. In fact, the grazing overlap between deer and wild horses is only 1%. While cattle and deer have a 4% grazing overlap.
The rewilding (and relocating) of wild horses into designated wilderness areas where deer and elk are currently suffering from collapsed populations, provides additional natural prey for apex predators (part of Natural Selection), which takes some of the depredation pressures off deer and elk, thereby rebalancing ecosystems.
More here: Foods of wild horses, deer, and cattle in the Douglas Mountain area, Colorado. Hansen, R. M., Clark, R. C., & Lawhorn, W. (1977). Journal of Range Management, 30(2), 116-118. https://repository.arizona.edu/handle/10150/646893
Wild Horse Fire Brigade is arguably a pragmatic plan that can be instituted under existing law, and benefits the majority of stakeholders around public land management and wild horses. Wild Horse Fire Brigade saves forests, wildlife, riparian areas, fisheries and wild horses.
Understanding ‘Wild Horse Fire Brigade’
The Supporting Science of Wildfire Grazing
‘William E. Simpson II – Wild Horse Ethologist’
References:
- Collapse of the world’s largest herbivores: “By altering the quantity and distribution of fuel supplies, large herbivores can shape the frequency, intensity, and spatial distribution of fires across a landscape”. William J. Ripple. Et. Al. http://advances.sciencemag.org/content/1/4/e1400103.full
- Rewilding: Jozef Keulartz. “The removal of large herbivores has adverse effects on landscape structure and ecosystem functioning. In wetter ecosystems, the loss of large herbivores is associated with an increased abundance of woody plants and the development of a closed-canopy vegetation. In drier ecosystems, reductions of large grazers can lead to a high grass biomass, and thus, to an increase in the frequency and intensity of wildfires. Together, with the loss of a prey base for large carnivores, these changes in vegetation structures and fire regimes may trigger cascades of extinctions (Bakker et al., 2016; Estes et al., 2011; Hopcraft, Olff, & Sinclair, 2009; Malhi et al., 2016).” http://oxfordre.com/environmentalscience/view/10.1093/acrefore/9780199389414.001.0001/acrefore-9780199389414-e-545
- Effects of large herbivores on fire regimes and wildfire mitigation. Julia Rouet-Leduc, Guy Pe’er, Francisco Moreira, Aletta Bonn, Wouter Helmer, Shahin A. A. Shahsavan Zadeh, Alexander Zizka, Fons van der Plas https://besjournals.onlinelibrary.wiley.com/doi/full/10.1111/1365-2664.13972
- Global response of fire activity to late Quaternary grazer extinctions. Allison T. Karp J. Tyler Faith Jennifer R. Marlon and A. Carla Staver
- G. R. van der Werf, J. T. Randerson, L. Giglio, T. T. van Leeuwen, Y. Chen, B. M. Rogers, M. Mu, M. J. E. van Marle, D. C. Morton, G. J. Collatz, R. J. Yokelson, P. S. Kasibhatla, Global fire emissions estimates during 1997-2016. Earth Syst. Sci. Data 9, 697–720 (2017).
- S. Archibald, C. E. R. Lehmann, C. M. Belcher, W. J. Bond, R. A. Bradstock, A.-L. Daniau, K. G. Dexter, E. J. Forrestel, M. Greve, T. He, S. I. Higgins, W. A. Hoffmann, B. B. Lamont, D. J. McGlinn, G. R. Moncrieff, C. P. Osborne, J. G. Pausas, O. Price, B. S. Ripley, B. M. Rogers, D. W. Schwilk, M. F. Simon, M. R. Turetsky, G. R. Van der Werf, A. E. Zanne, Biological and geophysical feedbacks with fire in the Earth system. Environ. Res. Lett. 13, 033003 (2018).
- C. N. Johnson, L. D. Prior, S. Archibald, H. M. Poulos, A. M. Barton, G. J. Williamson, D. M. J. S. Bowman, Can trophic rewilding reduce the impact of fire in a more flammable world? Phil. Trans. R. Soc. B 373, 20170443 (2018).
- S. Archibald, G. P. Hempson, Competing consumers: Contrasting the patterns and impacts of fire and mammalian herbivory in Africa. Phil. Trans. R. Soc. B 371, 20150309 (2016).
- A. C. Staver, J. O. Abraham, G. P. Hempson, A. T. Karp, J. T. Faith, The past, present, and future of herbivore impacts on savanna vegetation. J. Ecol. 109, 2804–2822 (2021).
- G. K. Charles, L. M. Porensky, C. Riginos, K. E. Veblen, T. P. Young, Herbivore effects on productivity vary by guild: Cattle increase mean productivity while wildlife reduce variability. Ecol. Appl. 27, 143–155 (2017).
- G. P. Hempson, S. Archibald, W. J. Bond, A continent-wide assessment of the form and intensity of large mammal herbivory in Africa. Science 350, 1056–1061 (2015).
- S. Jia, X. Wang, Z. Yuan, F. Lin, J. Ye, Z. Hao, M. S. Luskin, Global signal of top-down control of terrestrial plant communities by herbivores. Proc. Natl. Acad. Sci. U.S.A. 115, 6237–6242 (2018).
- M. S. Waldram, W. J. Bond, W. D. Stock, Ecological engineering by a mega-grazer: White Rhino impacts on a south African savanna. Ecosystems 11, 101–112 (2008).
- S. J. McNaughton, Grazing Lawns: Animals in Herds, Plant Form, and Coevolution. Am. Nat. 124, 863–886 (1984).
- E. S. Forbes, J. H. Cushman, D. E. Burkepile, T. P. Young, M. Klope, H. S. Young, Synthesizing the effects of large, wild herbivore exclusion on ecosystem function. Funct. Ecol. 33, 1597–1610 (2019).
- E. S. Bakker, J. L. Gill, C. N. Johnson, F. W. M. Vera, C. J. Sandom, G. P. Asner, J.-C. Svenning, Combining paleo-data and modern exclosure experiments to assess the impact of megafauna extinctions on woody vegetation. Proc. Natl. Acad. Sci. U.S.A. 113, 847–855 (2016).
- G. P. Hempson, S. Archibald, W. J. Bond, The consequences of replacing wildlife with livestock in Africa. Sci. Rep. 7, 17196 (2017).
- S. E. Koerner, M. D. Smith, D. E. Burkepile, N. P. Hanan, M. L. Avolio, S. L. Collins, A. K. Knapp, N. P. Lemoine, E. J. Forrestel, S. Eby, D. I. Thompson, G. A. Aguado-Santacruz, J. P. Anderson, T. M. Anderson, A. Angassa, S. Bagchi, E. S. Bakker, G. Bastin, L. E. Baur, K. H. Beard, E. A. Beever, P. J. Bohlen, E. H. Boughton, D. Canestro, A. Cesa, E. Chaneton, J. Cheng, C. M. D’Antonio, C. Deleglise, F. Dembélé, J. Dorrough, D. J. Eldridge, B. Fernandez-Going, S. Fernández-Lugo, L. H. Fraser, B. Freedman, G. García-Salgado, J. R. Goheen, L. Guo, S. Husheer, M. Karembé, J. M. H. Knops, T. Kraaij, A. Kulmatiski, M.-M. Kytöviita, F. Lezama, G. Loucougaray, A. Loydi, D. G. Milchunas, S. J. Milton, J. W. Morgan, C. Moxham, K. C. Nehring, H. Olff, T. M. Palmer, S. Rebollo, C. Riginos, A. C. Risch, M. Rueda, M. Sankaran, T. Sasaki, K. A. Schoenecker, N. L. Schultz, M. Schütz, A. Schwabe, F. Siebert, C. Smit, K. A. Stahlheber, C. Storm, D. J. Strong, J. Su, Y. V. Tiruvaimozhi, C. Tyler, J. Val, M. L. Vandegehuchte, K. E. Veblen, L. T. Vermeire, D. Ward, J. Wu, T. P. Young, Q. Yu, T. J. Zelikova, Change in dominance determines herbivore effects on plant biodiversity. Nat. Ecol. Evol. 2, 1925–1932 (2018).
- J. Rowan, J. T. Faith, in The Ecology of Browsing and Grazing II, I. J. Gordon, H. H. T. Prins, Eds. (Springer, 2019), pp. 61–79.
- E. J. Lundgren, D. Ramp, J. Rowan, O. Middleton, S. D. Schowanek, O. Sanisidro, S. P. Carroll, M. Davis, C. J. Sandom, J.-C. Svenning, A. D. Wallach, Introduced herbivores restore Late Pleistocene ecological functions. Proc. Natl. Acad. Sci. U.S.A. 117, 7871–7878 (2020).
- A. W. Illius, I. J. Gordon, Modelling the nutritional ecology of ungulate herbivores: Evolution of body size and competitive interactions. Oecologia 89, 428–434 (1992).
- J. L. Gill, J. W. Williams, S. T. Jackson, K. B. Lininger, G. S. Robinson, Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 326, 1100–1103 (2009).
- G. S. Robinson, L. P. Burney, D. A. Burney, Landscape paleoecology and megafaunal extinction in southeastern New York State. Ecol. Monogr. 75, 295–315 (2005).
- D. A. Burney, G. S. Robinson, L. P. Burney, Sporormiella and the late Holocene extinctions in Madagascar. Proc. Natl. Acad. Sci. U.S.A. 100, 10800–10805 (2003).
- S. Rule, B. W. Brook, S. G. Haberle, C. S. M. Turney, A. P. Kershaw, C. N. Johnson, The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science 335, 1483–1486 (2012).
- S. D. Schowanek, M. Davis, E. J. Lundgren, O. Middleton, J. Rowan, R. Ø. Pedersen, D. Ramp, C. J. Sandom, J.-C. Svenning, Reintroducing extirpated herbivores could partially reverse the late Quaternary decline of large and grazing species. Glob. Ecol. Biogeogr. 30, 896–908 (2021).
- E. J. Lundgren, S. D. Schowanek, J. Rowan, O. Middleton, R. Ø. Pedersen, A. D. Wallach, D. Ramp, M. Davis, C. J. Sandom, J.-C. Svenning, Functional traits of the world’s late Quaternary large-bodied avian and mammalian herbivores. Sci. Data 8, 17 (2021).
- J. R. Marlon, R. Kelly, A.-L. Daniau, B. Vannière, M. J. Power, P. Bartlein, P. Higuera, O. Blarquez, S. Brewer, T. Brücher, A. Feurdean, G. G. Romera, V. Iglesias, S. Y. Maezumi, B. Magi, C. J. Courtney Mustaphi, T. Zhihai, Reconstructions of biomass burning from sediment-charcoal records to improve data-model comparisons. Biogeosciences 13, 3225–3244 (2016).
- B. J. Enquist, A. J. Abraham, M. B. J. Harfoot, Y. Malhi, C. E. Doughty, The megabiota are disproportionately important for biosphere functioning. Nat. Commun. 11, 699 (2020).
- A. L. Daniau, P. J. Bartlein, S. P. Harrison, I. C. Prentice, S. Brewer, P. Friedlingstein, T. I. Harrison-Prentice, J. Inoue, K. Izumi, J. R. Marlon, S. Mooney, M. J. Power, J. Stevenson, W. Tinner, M. Andrič, J. Atanassova, H. Behling, M. Black, O. Blarquez, K. J. Brown, C. Carcaillet, E. A. Colhoun, D. Colombaroli, B. A. S. Davis, D. D’Costa, J. Dodson, L. Dupont, Z. Eshetu, D. G. Gavin, A. Genries, S. Haberle, D. J. Hallett, G. Hope, S. P. Horn, T. G. Kassa, F. Katamura, L. M. Kennedy, P. Kershaw, S. Krivonogov, C. Long, D. Magri, E. Marinova, G. M. McKenzie, P. I. Moreno, P. Moss, F. H. Neumann, E. Norström, C. Paitre, D. Rius, N. Roberts, G. S. Robinson, N. Sasaki, L. Scott, H. Takahara, V. Terwilliger, F. Thevenon, R. Turner, V. G. Valsecchi, B. Vannière, M. Walsh, N. Williams, Y. Zhang, Predictability of biomass burning in response to climate changes. Global Biogeochem. Cycles 26, GB4007 (2012).
- J. R. Marlon, P. J. Bartlein, M. K. Walsh, S. P. Harrison, K. J. Brown, M. E. Edwards, P. E. Higuera, M. J. Power, R. S. Anderson, C. Briles, A. Brunelle, C. Carcaillet, M. Daniels, F. S. Hu, M. Lavoie, C. Long, T. Minckley, P. J. H. Richard, A. C. Scott, D. S. Shafer, W. Tinner, C. E. Umbanhowar Jr., C. Whitlock, Wildfire responses to abrupt climate change in North America. Proc. Natl. Acad. Sci. U.S.A. 106, 2519–2524 (2009).
- A.-L. Daniau, M. F. Sánchez Goñi, P. Martinez, D. H. Urrego, V. Bout-Roumazeilles, S. Desprat, J. R. Marlon, Orbital-scale climate forcing of grassland burning in southern Africa. Proc. Natl. Acad. Sci. U.S.A. 110, 5069–5073 (2013).
- S. L. Cocker, M. F. J. Pisaric, F. M. G. McCarthy, J. C. Vermaire, P. Beaupre, L. C. Cwynar, Dung analysis of the East Milford mastodons: Dietary and environmental reconstructions from central Nova Scotia at ~75 ka yr BP. Can. J. Earth Sci. 58, 1059–1072 (2021).
- M. A. Krawchuk, M. A. Moritz, Constraints on global fire activity vary across a resource gradient. Ecology 92, 121–132 (2011).
- T. Andermann, S. Faurby, S. T. Turvey, A. Antonelli, D. Silvestro, The past and future human impact on mammalian diversity. Sci. Adv. 6, eabb2313 (2020).
- C. N. Johnson, Fire, people and ecosystem change in Pleistocene Australia. Aust. J. Bot. 64, 643–651 (2016).
- W. J. Ripple, T. M. Newsome, C. Wolf, R. Dirzo, K. T. Everatt, M. Galetti, M. W. Hayward, G. I. H. Kerley, T. Levi, P. A. Lindsey, D. W. Macdonald, Y. Malhi, L. E. Painter, C. J. Sandom, J. Terborgh, B. Van Valkenburgh, Collapse of the world’s largest herbivores. Sci. Adv. 1, e1400103 (2015).
- A. Pachzelt, M. Forrest, A. Rammig, S. I. Higgins, T. Hickler, Potential impact of large ungulate grazers on African vegetation, carbon storage and fire regimes. Glob. Ecol. Biogeogr. 24, 991–1002 (2015).
- N. Andela, D. C. Morton, L. Giglio, Y. Chen, G. R. van der Werf, P. S. Kasibhatla, R. S. DeFries, G. J. Collatz, S. Hantson, S. Kloster, D. Bachelet, M. Forrest, G. Lasslop, F. Li, S. Mangeon, J. R. Melton, C. Yue, J. T. Randerson, A human-driven decline in global burned area. Science 356, 1356–1362 (2017).
- C. E. Cordova, W. C. Johnson, An 18 ka to present pollen- and phytolith-based vegetation reconstruction from Hall’s Cave, south-central Texas, USA. Quat. Res. 92, 497–518 (2019).
- D. M. Nelson, M. A. Urban, A. P. Kershaw, F. S. Hu, Late-Quaternary variation in C3 and C4 grass abundance in southeastern Australia as inferred from δ13C analysis: Assessing the roles of climate, pCO2, and fire. Quat. Sci. Rev. 139, 67–76 (2016).
- P. T. Moss, G. B. Dunbar, Z. Thomas, C. Turney, A. P. Kershaw, G. E. Jacobsen, A 60000-year record of environmental change for the Wet Tropics of north-eastern Australia based on the ODP 820 marine core. J. Quaternary Sci. 32, 704–716 (2017).
- A. P. Kershaw, G. M. McKenzie, N. Porch, R. G. Roberts, J. Brown, H. Heijnis, M. L. Orr, G. Jacobsen, P. R. Newall, A high-resolution record of vegetation and climate through the last glacial cycle from Caledonia Fen, southeastern highlands of Australia. J. Quaternary Sci. 22, 481–500 (2007).
- R. A. Lopes dos Santos, P. De Deckker, E. C. Hopmans, J. W. Magee, A. Mets, J. S. Sinninghe Damsté, S. Schouten, Abrupt vegetation change after the Late Quaternary megafaunal extinction in southeastern Australia. Nat. Geosci. 6, 627–631 (2013).
- B. Hermanowski, M. L. da Costa, H. Behling, Environmental changes in southeastern Amazonia during the last 25,000 yr revealed from a paleoecological record. Quat. Res. 77, 138–148 (2012).
- B. Hermanowski, M. L. Da Costa, H. Behling, Possible linkages of palaeofires in southeast Amazonia to a changing climate since the Last Glacial Maximum. Veg. Hist. Archaeobot. 24, 279–292 (2014).
- D. M. Nelson, D. Verschuren, M. A. Urban, F. S. Hu, Long-term variability and rainfall control of savanna fire regimes in equatorial East Africa. Glob. Change Biol. 18, 3160–3170 (2012).
- L. Scott, Climatic conditions in Southern Africa since the last glacial maximum, inferred from pollen analysis. Palaeogeogr. Palaeoclimatol. Palaeoecol. 70, 345–353 (1989).
- L. Scott, J. C. Vogel, Late Quaternary pollen profile from the Transvaal Highveld, South Africa. S. Afr. J. Sci. 79, 266–272 (1983).
- M. A. Urban, D. M. Nelson, F. A. Street-Perrott, D. Verschuren, F. S. Hul, A late-Quaternary perspective, on atmospheric pCO2, climate, and fire as drivers of C4-grass abundance. Ecology 96, 642–653 (2015).
- N. Shi, L. M. Dupont, H. J. Beug, R. Schneider, Vegetation and climate changes during the last 21000 years in S.W. Africa based on a marine pollen record. Veg. Hist. Archaeobot. 7, 127–140 (1998).
- S. van der Kaars, X. Wang, P. Kershaw, F. Guichard, D. A. Setiabudi, A Late Quaternary palaeoecological record from the Banda Sea, Indonesia: Patterns of vegetation, climate and biomass burning in Indonesia and northern Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 155, 135–143 (2000).
- S. Faurby, M. Davis, R. Ø. Pedersen, S. D. Schowanek, A. Antonelli, J.-C. Svenning, PHYLACINE 1.2: The Phylogenetic Atlas of Mammal Macroecology. Ecology 99, 2626 (2018).
- J. Kingdon, D. Happold, T. Butynski, M. Hoffman, M. Happold, J. Kalina, Mammals of Africa, Volumes I-VI (Bloomsbury Publishing, 2013).
- J. O. Abraham, G. P. Hempson, A. C. Staver, Drought-response strategies of savanna herbivores. Ecol. Evol. 9, 7047–7056 (2019).
- S. C. Wang, C. R. Marshall, Estimating times of extinction in the fossil record. Biol. Lett. 12, 20150989 (2016).
- J. T. Faith, T. A. Surovell, Synchronous extinction of North America’s Pleistocene mammals. Proc. Natl. Acad. Sci. U.S.A. 106, 20641–20645 (2009).A. D. Barnosky, E. L. Lindsey, N. A. Villavicencio, E. Bostelmann, E. A. Hadly, J. Wanket, C. R. Marshall, Variable impact of late-Quaternary megafaunal extinction in causing ecological state shifts in North and South America. Proc. Natl. Acad. Sci. U.S.A. 113, 856–861 (2016).
- K. M. Wilson, M. G. Hill, Synthesis and assessment of the flat-headed peccary record in North America. Quat. Sci. Rev. 248, 106601 (2020).
- M. T. Boulanger, R. L. Lyman, Northeastern North American Pleistocene megafauna chronologically overlapped minimally with Paleoindians. Quat. Sci. Rev. 85, 35–46 (2014).
- J. M. Broughton, E. M. Weitzel, Population reconstructions for humans and megafauna suggest mixed causes for North American Pleistocene extinctions. Nat. Commun. 9, 5441 (2018).
- A. D. Barnosky, E. L. Lindsey, Timing of Quaternary megafaunal extinction in South America in relation to human arrival and climate change. Quat. Int. 217, 10–29 (2010).J. T. Faith, Late Pleistocene and Holocene mammal extinctions on continental Africa. Earth Sci. Rev. 128, 105–121 (2014).
- R. G. Klein, in Quaternary Extinctions: A Prehistoric Revolution, P. S. Martin, R. Klein, Eds. (Univ. Arizona Press, 1984), pp. 553–573.
- R. G. Roberts, T. F. Flannery, L. K. Ayliffe, H. Yoshida, J. M. Olley, G. J. Prideaux, G. M. Laslett, A. Baynes, M. A. Smith, R. Jones, B. L. Smith, New ages for the last Australian megafauna: Continent-wide extinction about 46,000 years ago. Science 292, 1888–1892 (2001).
- F. Saltré, M. Rodríguez-Rey, B. W. Brook, C. N. Johnson, C. S. M. Turney, J. Alroy, A. Cooper, N. Beeton, M. I. Bird, D. A. Fordham, R. Gillespie, S. Herrando-Pérez, Z. Jacobs, G. H. Miller, D. Nogués-Bravo, G. J. Prideaux, R. G. Roberts, C. J. A. Bradshaw, Climate change not to blame for late Quaternary megafauna extinctions in Australia. Nat. Commun. 7, 10511 (2016).
- S. van der Kaars, G. H. Miller, C. S. M. Turney, E. J. Cook, D. Nürnberg, J. Schönfeld, A. P. Kershaw, S. J. Lehman, Humans rather than climate the primary cause of Pleistocene megafaunal extinction in Australia. Nat. Commun. 8, 14142 (2017).
- S. Wroe, J. H. Field, M. Archer, D. K. Grayson, G. J. Price, J. Louys, J. T. Faith, G. E. Webb, I. Davidson, S. D. Mooney, Climate change frames debate over the extinction of megafauna in Sahul (Pleistocene Australia-New Guinea). Proc. Natl. Acad. Sci. U.S.A. 110, 8777–8781 (2013).
- D. J. Meltzer, Pleistocene Overkill and North American Mammalian Extinctions. Annu. Rev. Anthropol. 44, 33–53 (2015).
- A. J. Stuart, Late Quaternary megafaunal extinctions on the continents: A short review. Geol. J. 50, 338–363 (2015).
- F. Saltré, J. Chadoeuf, K. J. Peters, M. C. McDowell, T. Friedrich, A. Timmermann, S. Ulm, C. J. A. Bradshaw, Climate-human interaction associated with southeast Australian megafauna extinction patterns. Nat. Commun. 10, 5311 (2019).
- B. A. Leys, J. R. Marlon, C. Umbanhowar, B. Vannière, Global fire history of grassland biomes. Ecol. Evol. 8, 8831–8852 (2018).
- G. Levavasseur, M. Vrac, D. M. Roche, D. Paillard, Statistical modelling of a new global potential vegetation distribution. Environ. Res. Lett. 7, 044019 (2012).
- P. De Deckker, S. van der Kaars, S. Haberle, Q. Hua, J.-B. W. Stuut, The pollen record from marine core MD03-2607 from offshore Kangaroo Island spanning the last 125 ka; implications for vegetation changes across the Murray-Darling Basin. Aust. J. Earth Sci. 68, 928–951 (2021).
- J. L. Gill, Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169 (2014).
- A. C. Staver, W. J. Bond, W. D. Stock, S. J. van Rensburg, M. S. Waldram, Browsing and fire interact to suppress tree density in an African savanna. Ecol. Appl. 19, 1909–1919 (2009).
- D. M. Kimuyu, R. L. Sensenig, C. Riginos, K. E. Veblen, T. P. Young, Native and domestic browsers and grazers reduce fuels, fire temperatures, and acacia ant mortality in an African savanna. Ecol. Appl. 24, 741–749 (2014).
- J. L. Gill, J. W. Williams, S. T. Jackson, J. P. Donnelly, G. C. Schellinger, Climatic and megaherbivory controls on late-glacial vegetation dynamics: A new, high-resolution, multi-proxy record from Silver Lake, Ohio. Quat. Sci. Rev. 34, 66–80 (2012).
- J. L. Commerford, K. K. McLauchlan, S. Sugita, Calibrating Vegetation Cover and Grassland Pollen Assemblages in the Flint Hills of Kansas, USA. Am. J. Plant Sci. 4, 1–10 (2013).
- M. J. Power, J. R. Marlon, P. J. Bartlein, S. P. Harrison, Fire history and the global charcoal database: A new tool for hypothesis testing and data exploration. Palaeogeogr. Palaeoclimatol. Palaeoecol. 291, 52–59 (2010).
- O. Blarquez, B. Vannière, J. R. Marlon, A.-L. Daniau, M. J. Power, S. Brewer, P. J. Bartlein, Paleofire: An R package to analyse sedimentary charcoal records from the Global Charcoal Database to reconstruct past biomass burning. Comput. Geosci. 72, 255–261 (2014).
- M. J. Power, J. Marlon, N. Ortiz, P. J. Bartlein, S. P. Harrison, F. E. Mayle, A. Ballouche, R. H. W. Bradshaw, C. Carcaillet, C. Cordova, S. Mooney, P. I. Moreno, I. C. Prentice, K. Thonicke, W. Tinner, C. Whitlock, Y. Zhang, Y. Zhao, A. A. Ali, R. S. Anderson, R. Beer, H. Behling, C. Briles, K. J. Brown, A. Brunelle, M. Bush, P. Camill, G. Q. Chu, J. Clark, D. Colombaroli, S. Connor, A.-L. Daniau, M. Daniels, J. Dodson, E. Doughty, M. E. Edwards, W. Finsinger, D. Foster, J. Frechette, M.-J. Gaillard, D. G. Gavin, E. Gobet, S. Haberle, D. J. Hallett, P. Higuera, G. Hope, S. Horn, J. Inoue, P. Kaltenrieder, L. Kennedy, Z. C. Kong, C. Larsen, C. J. Long, J. Lynch, E. A. Lynch, M. McGlone, S. Meeks, S. Mensing, G. Meyer, T. Minckley, J. Mohr, D. M. Nelson, J. New, R. Newnham, R. Noti, W. Oswald, J. Pierce, P. J. H. Richard, C. Rowe, M. F. Sanchez Goñi, B. N. Shuman, H. Takahara, J. Toney, C. Turney, D. H. Urrego-Sanchez, C. Umbanhowar, M. Vandergoes, B. Vanniere, E. Vescovi, M. Walsh, X. Wang, N. Williams, J. Wilmshurst, J. H. Zhang, Changes in fire regimes since the last glacial maximum: An assessment based on a global synthesis and analysis of charcoal data. Clim. Dyn. 30, 887–907 (2008).
- R. S. Vachula, N. Richter, Informing sedimentary charcoal-based fire reconstructions with a kinematic transport model. Holocene 28, 173–178 (2017).
- J. R. Marlon, P. J. Bartlein, D. G. Gavin, C. J. Long, R. S. Anderson, C. E. Briles, K. J. Brown, D. Colombaroli, D. J. Hallett, M. J. Power, E. A. Scharf, M. K. Walsh, Long-term perspective on wildfires in the western USA. Proc. Natl. Acad. Sci. U.S.A. 109, E535–E543 (2012).
- J. Marlon, P. J. Bartlein, C. Whitlock, Fire-fuel-climate linkages in the northwestern USA during the Holocene. Holocene 16, 1059–1071 (2006).
- P. E. Higuera, B. N. Shuman, K. D. Wolf, Rocky Mountain subalpine forests now burning more than any time in recent millennia. Proc. Natl. Acad. Sci. U.S.A. 118, e2103135118 (2021).
- K. Pearson, On lines and planes of closest fit to systems of points in space. Lond. Edinb. Dublin Philos. Mag. J. Sci. 2, 559–572 (1901).
- H. Akaike, A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19, 716–723 (1974).
- Cattle Grazing Effects on Macroinvertebrates in an Oregon Mountain Stream; Rangeland Ecology and Management 60(3), 293-303, (1 May 2007) James D. McIver and Michael L. McInnis; https://doi.org/10.2111/1551-5028(2007)60[293:CGEOMI]2.0.CO;2
- Influence of ruminant digestive processes on germination of ingested seeds; https://ir.library.oregonstate.edu/concern/graduate_thesis_or_dissertations/v405sg230
- Knopff KH, Knopff AA, Kortello A, Boyce MS. (2010). Cougar Kill Rate and Prey Composition in a Multiprey System. Journal of Wildlife Management 74(7):000–000; 2010; DOI: 10.2193/2009-314. Downloaded at: http://sci-northern.ab.ca/wp-content/uploads/2010/12/CougarKillRateandPreyComposition.pdf
- French, Brett. (2010, December 9). Ferocious appetites: Study finds mountain lions may be eating more than previously believed. Billings Gazette. Retrieved from:
http://billingsgazette.com/lifestyles/recreation/article_d9cf046b-2c47-539f-a267-972e72e570b6.html - Turner JW Jr and Morrison ML. (2001). Influence of Predation by Mountain Lions on Numbers and Survivorship of a Feral Horse Population. The Southwestern Naturalist. Vol. 46, No. 2 (Jun., 2001), pp. 183-190. Available at:
http://www.jstor.org/discover/10.2307/3672527?uid=2129&uid=2&uid=70&uid=4&sid=21101018535373 - Greger, Paul D. and Romney, Evan M. (1999). High foal mortality limits growth of a desert feral horse population in Nevada. Great Basin Naturalist: Vol. 59: No. 4, Article 10. Available at: https://scholarsarchive.byu.edu/gbn/vol59/iss4/10
- French, Brett. (2004, August 12). Lions blamed for deaths of Pryor foals. Billings Gazette. Retrieved from:
http://billingsgazette.com/news/state-and-regional/montana/lions-blamed-for-deaths-of-pryor-foals/article_ab0b2389-31a1-5110-8fb5-a21e9f753de7.htm
